Featured
Latest News

April 24, 2024
add comment
The Kaduna State House of Assembly has launched an investigation into the financial dealings of the state...

BREAKING: Emeka lhedloha Dumps PDP
April 23, 2024
1 comment

April 24, 2024
add comment
Megan Thee Stallion forced her cameraman to watch her having sex in a moving car, according to...

Producer Calls Out Davido Over Unpaid Royalties
April 23, 2024
add comment

April 24, 2024
add comment
Lagos State Governor, Babajide Sanwo-Olu, today welcomed soon-to-be Guinness World Record holder, Tunde Onakoya, back to Lagos....

Nobody Can Give Me Headache – Yahaya Bello (Video)
April 24, 2024
add comment

World cup: Super Eagles to play South Africa June 7
April 13, 2024
add comment

April 24, 2024
add comment
The US Senate has voted overwhelmingly to provide a USD 95.3 billion aid package to Ukraine and...

First female transgender mosque opens in Bangladeshi
April 22, 2024
add comment

April 24, 2024
add comment
The Minister of Aviation, Festus Keyamo has suspended Dana Airline operations in the country. The directive followed...

CBN Sells Fresh Dollars To BDCs At ₦1,021/$
April 23, 2024
add comment

Naira depreciates to N1,250/$ in parallel market
April 23, 2024
add comment

Firstbank Appoints Olusegun Alebiosu As New MD/MD
April 22, 2024
add comment

April 24, 2024
add comment
By Christian ABURIME The recent claims by the Anambra State Chairman of the Nigerian Union of Journalists,...

CS2 Gaming Sites
April 23, 2024
add comment

Kwankwaso’s Plot to Oust Ganduje
April 23, 2024
add comment

April 14, 2024
add comment
The National Drug Law Enforcement Agency has announced the discovery of a dangerous drug concoction named “Combine.”...
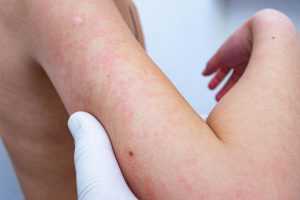
Jigawa confirms outbreak of meningitis in 6 LGs
March 4, 2024
add comment

April 24, 2024
add comment
Punish my abusers within 48 hours or face lawsuit – Student bullied by her classmates threaten school...

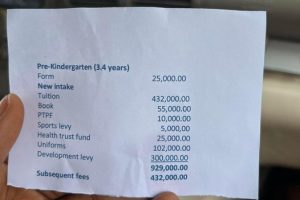
BREAKING: Lead British School Temporarily Closed
April 23, 2024
add comment

Female corper shows off location posted for NYSC
April 20, 2024
add comment